Recently, Prof. Qianghui Zhou and co-workers reported a cooperative catalytic system comprising of a PdII/XPhos complex and the potassium salt of 5-norbornene-2-carboxylic acid to enable the use of epoxides as alkylating reagents in the Catellani reaction. Related results were published in Angew. Chem. Int. Ed. (2018, 57, 3444-3448), which has been chosen as a “Front Cover”. The picture shows the key elements of this novel transformation, with representative buildings of Wuhan University in the background. Cover design by Jun Deng, Wuhan University.

This paper titled “Epoxides as Alkylating Reagents for the Catellani Reaction”. Dr. Hong-Gang Cheng is the first author, Prof. Qianghui Zhou is the only corresponding author, and Wuhan University is the only institution. Notably, Han Chen, Ruiming Chen, Zhi Geng, Jinyang Zhang and Yuming Zhang, who are undergraduates in the College of Chemistry and Molecular Sciences, contributed a lot in this research. This research was supported by the National Key Research and Development Program of China (No. 2016YFB0101203), the national “1000-Youth Talents Plan”, the Innovation Team Program of Wuhan University (No. 2042017kf0232), and start-up funding from Wuhan University.
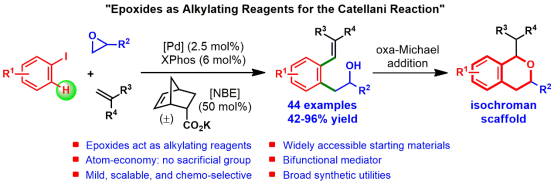
The Catellani-type reaction is a powerful strategy that allows the expeditious synthesis of highly substituted arenes. It utilizes synergistic interplay of palladium and 2-norbornene (NBE) catalysis to facilitate sequential ortho C–H functionalization and ipso termination of aryl iodides. Seminal work by the group of Catellani, followed by Lautens and others have significantly enriched this chemistry. Recent efforts in this area have expanded the scope of terminating reagents substantially as a gamut of functionalities can be appended at the ipso-position. In contrast, the electrophilic reagents developed for ortho C–H functionalization are quite limited.
To circumvent this challenge, the team of Prof. Qianghui Zhou developed a cooperative catalytic system comprising a PdII complex, XPhos and potassium salt of 5-norbornene-2-carboxylic acid that enables the use of epoxides as alkylating reagents in the Catellani reaction, thereby expanding the existing paradigm of this powerful transformation. The salient features of the strategy include its simply operation, mild reaction conditions, widely accessible starting materials, broad substrate scope and its high atom economy–since epoxide can be incorporated into the product in its entirety, without incurring any sacrificial groups. In addition, the products are synthetic useful intermediates, which can be rapid transformed to isochroman scaffolds via a subsequent oxo-Michael addition, the resulting isochroman substructure exists in many bioactive natural products and therapeutic agents (e.g., penicitrinol F, mevashuntin, dopamine D4 receptor antagonist U-101387, and 5-HT1D agonist PNU-109291 etc.). Currently, their developed method has been applied in the synthesis of methylated analogue of D4 agonist U-101387 and the core structure of penicitrinol F.
Paper Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201800573/full
Homepage of Prof. Qianghui Zhou’s Group: http://qhzhou.whu.edu.cn
Chinese Version: http://news.whu.edu.cn/info/1002/50530.htm
http://www.chem.whu.edu.cn/info/1312/6235.htm
