Ying Wang, Yao Yang, Shuangfeng Jia, Xiaoming Wang, Kangjie Lyu, Yanqiu Peng, He Zheng, Xing Wei, Huan Ren, Li Xiao, Jianbo Wang, David A. Muller, Héctor D. Abruña, Bing Joe Hwang, Juntao Lu & Lin Zhuang
Nat. Commun. 2019, 10, 1506.
DOI: 10.1038/s41467-019-09503-4
Abstract:
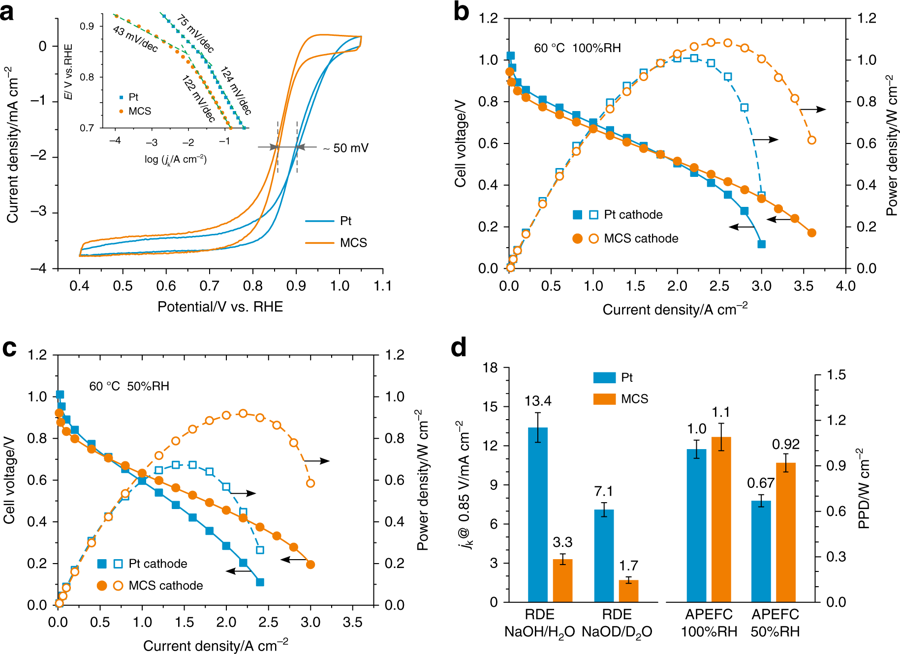
Alkaline polymer electrolyte fuel cells are a class of fuel cells that enable the use of non-precious metal catalysts, particularly for the oxygen reduction reaction at the cathode. While there have been alternative materials exhibiting Pt-comparable activity in alkaline solutions, to the best of our knowledge none have outperformed Pt in fuel-cell tests. Here we report a Mn-Co spinel cathode that can deliver greater power, at high current densities, than a Pt cathode. The power density of the cell employing the Mn-Co cathode reaches 1.1 W cm−2 at 2.5 A cm−2 at 60 oC. Moreover, this catalyst outperforms Pt at low humidity. In-depth characterization reveals that the remarkable performance originates from synergistic effects where the Mn sites bind O2 and the Co sites activate H2O, so as to facilitate the proton-coupled electron transfer processes. Such an electrocatalytic synergy is pivotal to the high-rate oxygen reduction, particularly under water depletion/low humidity conditions.
Full Text: https://www.nature.com/articles/s41467-019-09503-4
