(Correspondent: Rui-Wen Zhang) On June 26, Professor Hao Yin's work group from Wuhan University Medical Research Institute , Zhongnan Hospital Medical Research Institute, Ministry of Education's Frontier Science Center for Immunology and Metabolion, and Taikang Center for Life and Medical Sciences published a research paper in Cell entitled Amplification editing enables Amplification editing enables efficient and precise duplication of DNA from short sequence to megabase and chromosomal scale. The first author of the paper is Ruiwen Zhang, Zhou He, Yajing Shi, Xiangkun Sun, Xinyu Chen and Wang Guoquan, all of whom are graduate students at Wuhan University Medical Research Institute. Hao Yin is the independent corresponding author. Wuhan University is the signature unit of the first author.

Gene amplification (gene duplication) is the copying of DNA segments in an organism to form repetitive sequences. Amplification ranges from a few base pairs to large regions of the chromosome. Gene amplification is a structural variation of chromosomes that plays an important role in evolution, hereditary diseases, and the development of cancer. Existing gene editing tools are capable of precise editing of individual loci, but there is a lack of efficient and accurate genome structure editing tools. In this project, a method called Amplification Editing (AE) was developed, which can precisely and efficiently replicate genomic sequences from small fragments to the chromosome level in a programmable manner. It is a highly efficient and precise genome structure editing tool, which can extend the scope of precise replication from a single gene locus to the chromosome level.
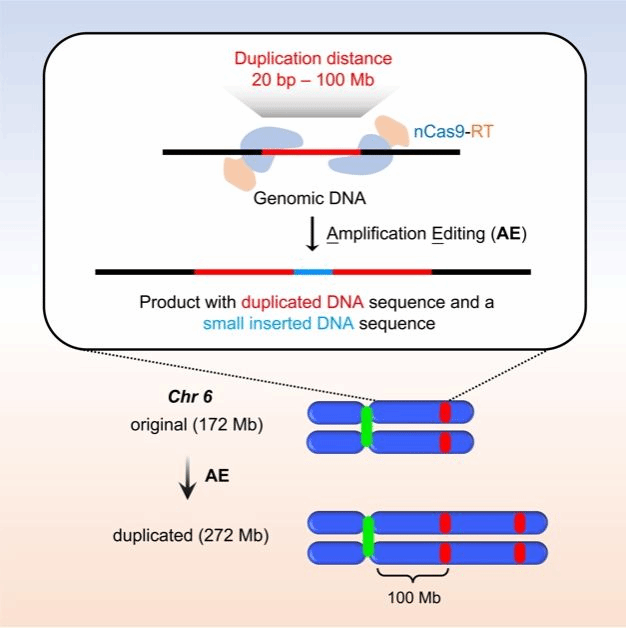
Mock-up of gene amplification performed by AE
The group validated this at multiple loci in 11 cell lines. In terms of AE replication length and efficiency, when the length of replicated DNA is 20 bp to 8 Kb, AE can replicate multiple times to form tandem repeat sequences. When the replication length was 1 Mb, the editing efficiency of AE was up to 73.0%; when the replication length was 100 Mb (close to the average length of human chromosomes), the efficiency reached 3.4%. The replication of chromosomal regions can be directly observed through experiments such as in situ fluorescence hybridization, karyotype analysis, and whole genome sequencing. In terms of the precision of AE editing, the second-generation sequencing at the junction and the full-length third-generation sequencing data all showed that indels is below 1%.
The group utilized AE to precisely correct the green fluorescent protein sequence and achieve endogenous overexpression of miRNA. Meanwhile, the group established an α-thalassemia model in myeloid leukemia cell line K562 and tested the effect of AE, achieving an increase in the mRNA level of HBA gene and the ratio of α/γ-bead protein. These results indicate that AE can restore gene expression, amplify miRNA and increase α-bead protein expression in the model cell line.
AE achieved efficient and precise short fragment and Mb-level editing at multiple loci in both human and murine stem cells, modeled genotypes of chromosomal microduplication disorders, and verified the precision of editing using whole genome sequencing. This demonstrates that AE is capable of accurately constructing oversized fragment duplication-associated disease models.
This study provides a preliminary investigation of the mechanism of AE. In RPE-1 cells that were inhibited from cell cycle, the efficiency of AE was reduced. In mouse primary neurons, AE could only achieve replication of short segments. This suggests that AE replication at the Mb level may depend on cell cycle.
In summary, the study has developed a genome editing tool called "Amplification Editing(AE)", which can realize precise replication from short fragments to chromosome lengths. The development of this tool can facilitate the establishment of genomic disease models and promote the research on gene evolution and cancer mechanisms.

Left to right: Zhang Ruifen, Wang Guoquan, Sun Xiangkun, Yin Hao, He Zhou, Shi Yajing, Chen Xinyu
It is reported that the work was funded by the National Natural Science Foundation of China and the Key Research and Development Program of the Ministry of Science and Technology. It is also supported by the Instrument and Equipment Sharing Core Facility of Wuhan University Institute of Medical Research and Zhongnan Hospital of Wuhan University.
Link to paper: https://doi.org/10.1016/j.cell.2024.05.056
Rewritten by Yiting Zhou.