Recently, Nature Communications published online the latest findings on the synthesis of fluoroalkyl alcohols via 1-silyl fluoroethanol by Professor Shen Xiao’s research group from the Institute for Advanced Studies, Wuhan University. The 1- (phenyl dimethylsilyl) -fluoroethanol developed by the team was used as the fluoroethanolization reagent, and the allylation, alkylation and alkenization reactions were realized efficiently and conveniently.
The article is entitled Direct Transfer of Tri- and di-fluoroethanol Units Enabled by Radical Activation of Organosilicon Reagents. Prof. Shen Xiao is the corresponding author of the article, and two co-first authors are Chen Xiang, a postdoctoral fellow and Gong Xingxing, a postgraduate, both from the Institute of Advanced Studies, WHU.
Fluoroalkyl alcohols are important structural units of many bioactive compounds, such as the drug molecule Befloxatone and the anticancer reagent Z (Figure 1). Moreover, fluoroalkyl alcohols are also an important organic synthesis intermediate, which is used to synthesize a series of useful organo-fluorine compounds. The traditional methods for obtaining fluoroalkyl tertiary alcohols are generally the nucleophilic addition reaction of aldehydes with fluoroalkyl reagents and the reduction of fluorine-containing ketones. However, due to the poor selectivity and restricted substrate applicability, these methods play a limited role in the synthesis of complicated fluorinated compounds. Thus, a different approach with better selectivity and wider application to constructing fluoroalkyl alcohols is supposed to appear urgently.
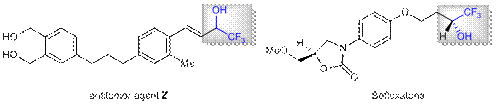
Figure 1 Drug molecule Befloxatone and anti-cancer reagent Z
Prof. Shen Xiao and his research group designed and successfully developed two fluoroalkyl organosilicon reagents:1-dimethyl(phenyl)silyl-2,2,2-trifluoroethanol and 1-dimethy(phenyl)silyl-2,2-difluoroethanol. In the presence of manganese catalyst, new carbon radicals were obtained by oxo-manganese bond homing and radical Brook rearranging, which react with allyl sulfone, α, β-unsaturated acyl aromatic amines or cinnamic acid derivatives to efficiently synthesize a series of fluorinated homoallyl alcohol, fluorinated alkyl alcohol or fluorinated allylic alcohols (Figure 2). Their findings realize the direct transfer reaction of trifluoroethanol and difluoroethanol unit, highlighting its broad substrate applicability and good functional group tolerance. The approach can be effectively applied to the late course functionalization of complex molecules or natural compounds, which has potential applicable value in the synthesis of natural materials and drug molecules.
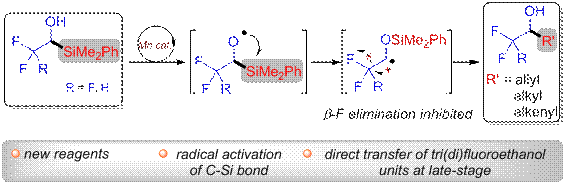
Figure 2 Efficient synthesis of fluorine-containing alcohol compounds
Article link: https://www.nature.com/articles/s41467-020-16380-9
DOI:10.1038/s41467-020-16380-9
Written by: Xie Zhangbin
Rewritten by: Zhu TongEdited by: Wu Buer, Sylvia and Hu Sijia
Edited by: Wu Buer