On February 23th, PNAS published online the research paper revealing the molecular mechanism that couples histone H2B ubiquitination to DNA replication and repair by the research group of Prof. Chen Xuefeng from the Department of Genetics in the College of Life Sciences of Wuhan University (WHU) and Hubei Key Laboratory of Cell Homeostasis.
The article was entitled RPA-mediated recruitment of Bre1 couples histone H2B ubiquitination to DNA replication and repair. PHD student Liu Guangxue (College of Life Sciences, WHU) is the first author, and Prof. Chen Xuefeng is the corresponding author. Prof. Huang Jun’s team (College of Life Sciences, Zhejiang University) also joined the research. It was supported by the General Program of the National Natural Science Foundation of China.
Maintenance of genome stability is essential to the normal proliferation and division of cells. Abnormalities in DNA replication or repair lead to gene mutations and genome instability, causing carcinogenesis or death of cells. In the past, Chen’s research group and studies at home and abroad found that histone H2B ubiquitination modification is important for promoting DNA replication and repair, and maintaining genome stability. But it is not clear how H2B ubiquitination, as an independent epigenetic modification, responds to DNA replication and repair.
Using the organism saccharomyces cerevisiae as a model, this study found that Bre1, an E3 ligase mediating H2B ubiquitination modification, was able to directly bind to the DNA replication and repair protein RPA through mass spectrometry. The RPA complex has the activity to naturally combine single-stranded DNA on chromatin and is indispensable for DNA replication and repair. The authors accurately located key amino acid sites mediating Bre1-RPA interaction and illustrated that these sites are highly conserved in evolution. Utilizing separation of function mutants, researchers demonstrated that RPA is responsible for recruiting Bre1 to sites of replication or breaks during DNA replication and repair, stimulating H2B ubiquitination modification on local chromatin, thereby facilitating the replication and repair. Disruption of the interaction between Bre1 and RPA results in delayed DNA replication, defects in response to replication stress, impaired homologous recombination repair, and a series of severe outcomes, including replication fork instability, increased spontaneous DNA mutations, more chromosome loss, and sensitivity to DNA damaging drugs. Therefore, the authors concluded that RPA mediated Bre1 recruitment couples H2B ubiquitination and DNA replication and repair to ensure proper cell replication and to maintain genome stability.
Finally, the authors realized that RNF20, the human homolog of Bre1, also interacts with the RPA complex, implicating that this mechanism is conserved in human cells. This study answers the important scientific question of how H2B ubiquitylation modification responds to DNA replication and repair, revealing a new function of RPA to control spatiotemporal distribution of chromatin with epigenetic modifications, and providing a theoretical reference for further investigation of related mechanisms in human cells.
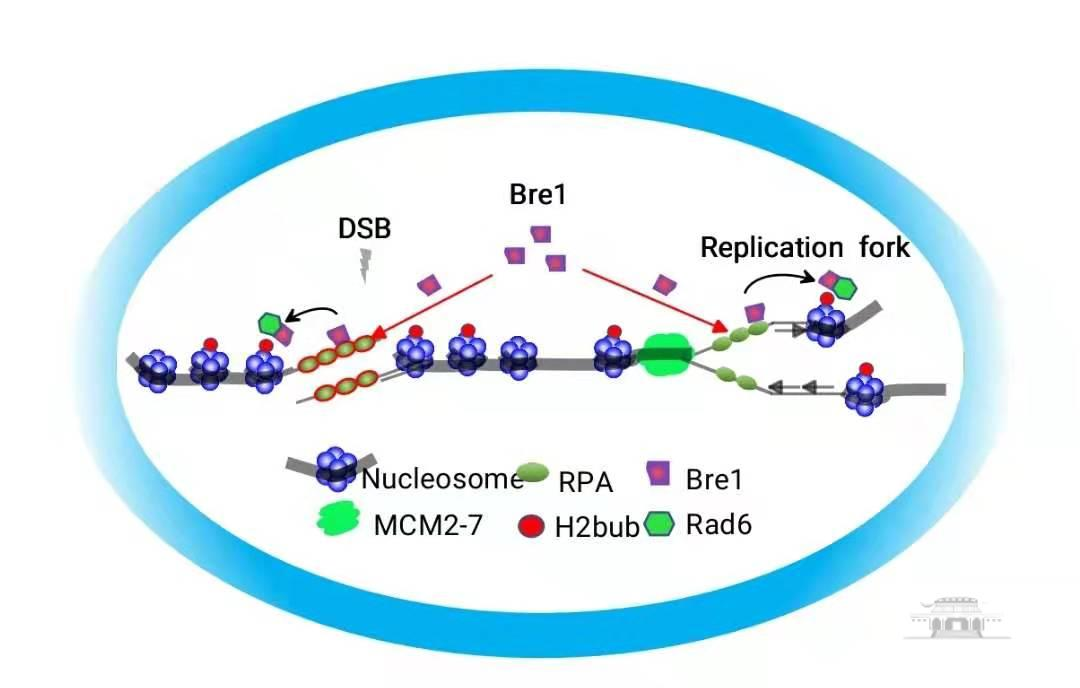
RPA-mediated recruitment of Bre1 ubiquitin ligase couples H2B ubiquitination to DNA replication and repair
Link to the paper: https://doi.org/10.1073/pnas.2017497118
Rewritten by Dong Xiaoqian
Edited by Yin Xiaoxue