On August 2, Cell Research, a sub-journal of Nature, published online the latest findings of Professor Luo Mengcheng’s research team (School of Basic Medical Sciences, Wuhan University). Employing an innovative proteomics approach and identifying a novel male sex-determining gene, SDX, on the X chromosome, the study provides a new theoretical basis for understanding the mechanism of mammalian sex determination and the treatment of human sex reversal diseases.
The paper is entitled SDX on the X chromosome is required for male sex determination. The first author is Zhan Junfeng, a doctoral student of clinical medicine in the Department of Medicine enrolled in 2014. Luo Mengcheng is the corresponding author.
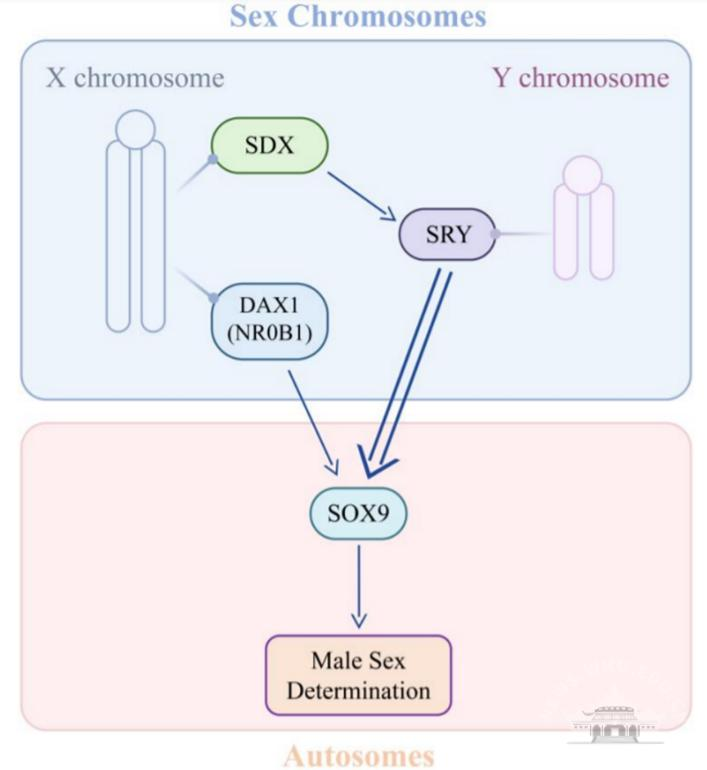
Model of SDX in male sex determination
Sex determination is one of the most fundamental biological development processes, as sex is the most important identity trait in humans. In most mammals, biological sex is determined by differences in sex chromosomes, for the autosomes are identical in both male and female. During the past 30 years, two genetic pathways of sex determination have been revealed in mammals: the testis-determining (SRY-SOX9-FGF9) and ovary-promoting (RSPO1-WNT/β-catenin-FOXL2) pathways. Previous studies have shown that SRY is the only gene in the Y chromosome necessary for sex determination. Among sex-reversed patients, about 15% of 46 XY females can be attributed to mutations of SRY. However, it remains elusive whether there is another sex chromosome gene necessary for sex determination. Interestingly, the African pygmy mouse Mus minutoides showed XY-female sex reversal due to the disruption of unknown genes on the X chromosome. The result indicates that male sex-determining genes probably exist on the X chromosome of mammals.
The research improved the method for screening and identifying chromatin-binding proteins. Previously, many key germ cell meiosis regulators were screened through other methods, including MEIOB, YTHDC1/2, etc. Identifying gonadal-related chromatin-binding protein for 11.5 days in the mouse embryonic period (E11.5, the critical period of sex determination), and considering the previous omics data on meiotic chromatin-binding protein, the team discovered a candidate gene that may regulate embryonic sex differentiation: 9430086K21Rik (also known as PWWP3B or MUM1L1). Based on its unique function in sex determination, they renamed the gene as SDX (Sex-Determining gene on the X chromosome).
First, the researchers analyzed the expression pattern of SDX during sex determination. The results showed that SDX expressed as early as in the initiation of sex determination and was localized predominantly in the nuclei of somatic cells in E11.5 and E13.5 male gonads. Moreover, SDX co-localized with SOX9 in Sertoli cells at both embryonic and postnatal testis. In contrast, no SDX protein was detected in the embryonic or postnatal ovaries. These data demonstrate that SDX may play a critical role in sex determination and maintaining functions of Sertoli cells.
To reveal the biological function of SDX in sex determination and take the possible death of SDX gene knockout into consideration, the team used a regular background mouse model, C57BL/6 (B6), to generate the conditional knockout mice. Then they obtained the complete SDX knockout mice (SDX–/Y) using a germ cell-specific cre transgene. Phenotypic analysis showed that approximately 25% (5 out of 20) of SDX–/Y adult mice were completely sex-reversed with the development of a normal reproductive system, while unreversed SDX–/Y males showed spermatogenic defects to different extents with age.
Next, they performed transcriptome analysis for the SDX+/Y and SDX–/Y gonads during male sex differentiation. Expression levels of SRY and its downstream target gene SOX9 were significantly reduced in the SDX–/Y gonads. SRY was reduced to approximately 30% of the level of the wild-type control during the period of sex determination. In the SDX–/Y gonads at E13.5, expression levels of Sox9 and AMH were also significantly lower than those in the SDX+/Y male gonads, indicating an obstacle in the male development pathway. Further immunofluorescence experiments on the protein level also confirmed a significant decrease in the expression level of SOX9/AMH. Through conservative analysis of the protein sequence, they discovered that SD0X is highly conserved in humans and mice. They further immuno-stained SOX9 and SDX in human testis and found that SDX completely co-localized with SOX9 in human Sertoli cells, which is the same as that in mice. These data strongly imply that SDX plays a crucial role in regulating and controlling human sex determination.
Based on relevant studies, the group proposed a new model of how sex chromosome genes determine sex differentiation in mammals. As a commander of the mammalian sex determination, the X and Y chromosomes together set the direction and coordinate with the autosomes in male or female development. The decision is mainly made by SRY on the Y chromosome and SDX on the X chromosome. SDX gives a strong signal for male sex determination by promoting SRY expression. It is commonly recognized that the Y chromosome (SRY) determines mammalian sex, and individuals develop as females by default without SRY. However, the research of Luo Mengcheng’s team suggests that SDX on the X chromosome is required to cooperate with SRY to assure male sex determination. Without the SDX gene on the X chromosome, the Y chromosome cannot guarantee that the individual will develop into a male.
Luo Mengcheng returned to China in 2016 to establish a reproductive development laboratory, whose research focuses on mammalian sex determination and meiosis. He is committed to revealing the genetic mechanism of human reproductive development defects and providing new theories and methods for the diagnosis and treatment of infertility. During his postdoctoral period, Luo Mengcheng’s research work on the MEIOB gene has been widely recognized in the field. The MEIOB gene was included in the 2020 version of the consensus of male reproduction-related gene testing experts and has been widely applied in clinical practice as one of the non-obstructive azoospermia detection genes.
Paper link: SDX on the X chromosome is required for male sex determination | Cell Research (nature.com)
Translated by: Dong Xiaoqian
Edited by: Wang Xuanqi