The Journal of the American Chemical recently published a research article named “Epoxide Electroreduction” and recommended it on its cover. The article reported the research co-conducted by two research groups from Wuhan University (WHU). One group was led by Professor Qi Xiaotian from the College of Chemistry and Molecular Sciences, and the other was led by Professor Lu Qingquan from The Institute for Advanced Studies. Both Qi Xiaotian and Lu Qingquan were corresponding authors.
The anti-Markovnikov alcohols have a wide range of applications in bulk/fine chemicals, agricultural chemicals, pharmaceuticals and spice chemistry. Selective hydrogenation of epoxides via heterogeneous or homogeneous catalysis can be used for the preparation of alcohols (Figure 1A). However, such reactions are of poor selectivity and usually require high temperature and high-pressure hydrogen, which may lead to high cost and result in dangerous consequences. In addition, ring-opening of epoxides mediated by alkali metals or catalyzed by titanocene also provides a free radical pathway for the preparation of alcohols. However, the use of equivalent reductants (e.g., Li, Ca) and hydrogen donors (1, 4-cyclohexadiene, Bu3SnH, or tert-butyl mercaptan) that are toxic and expensive has limited its industrial applications. Therefore, it is imperative to develop a hydrogen transfer reagent that is inexpensive, safe, and easy-to-operate.
Qi Xiaotian’s group has been devoted to the study of the mechanism of free radical chemical reactions. The theoretical calculation reveals the key factors affecting the activity and selectivity of free radical bond formation and breaking, thus precisely regulating product selectivity. Lu Qingquan’s group has focused on the core topic of "transformation and synthesis of alkyl molecules", emphasizing the large-scale preparation of high-value molecules through the development of new methods and synthesis processes by means of organic electroreduction, transition metal catalysis, bioelectrochemistry, etc. The combination between theoretical calculation and electrochemical synthesis has laid a theoretical foundation for the control of the selective hydrogenation of epoxides. The authors have proposed an electrochemical approach using electrons as a sustainable and safe redox reagent, promising regioselective electrohydrogenation of epoxides. Based on the law of mass and electron conservation, the authors have proposed that H2 is equal to two protons plus two electrons, i.e. H2 = 2H++ 2e−. Under electrochemical conditions, protons and electrons are directly used as potential hydrogen sources, avoiding the use of high-pressure hydrogen and transition metals, and effectively achieving selective electrohydrogenation of epoxy compounds (Figure 1a).
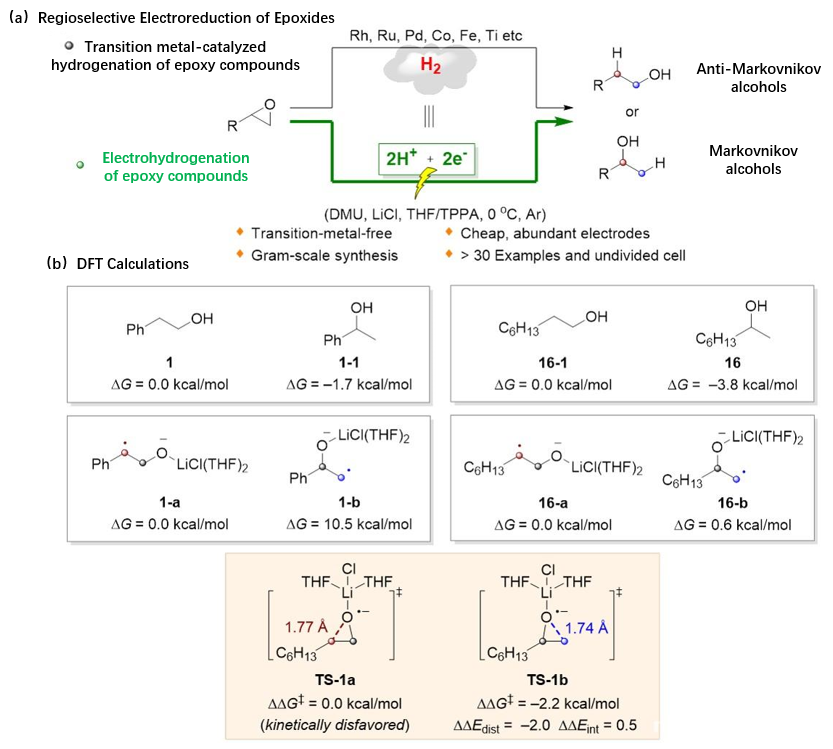
Figure 1 Study on electroreduction and reaction mechanism of epoxides
Based on a series of experiments and theoretical calculations, the possible mechanism of the reaction has been proposed. It has been proved that the hydrogen source of the reaction was 1, 3-dimethylurea (DMU), and TPPA could be used as not only a cosolvent to improve the solubility of electrolyte (LiCl) but also a key role in effectively stripping magnesium anodes and activating epoxy compounds. Density functional theory (DFT) calculations have further elucidated the selective control factors for electrohydrogenation of epoxides (Figure 1b). Calculation results have shown that inverse markovini electrohydrogenation of aryl-substituted epoxies derives from thermodynamic stability of benzyl radical intermediates, and markovial electrohydrogenation of alkyl-substituted epoxies depends on kinetic factor-driven markovial ring-opening process, namely, thermodynamic and kinetic factors jointly control the selectivity of electrohydrogenation of different epoxy compounds.
In conclusion, a mild and environmentally friendly electrohydrogenation method has been developed to achieve regioselective ring-opening of epoxides through close cooperation between theoretical calculation and electrochemistry. Various kinds of anti-martensite alcohol and martensite alcohol can be prepared by using electrons and protons as hydrogen sources, and the regioselectivity of hydrogenation can be regulated according to the results of mechanism study. This research project has been strongly supported by institutions such as the National Natural Science Foundation of China and the Supercomputing Center of WHU.
Link to the article: https://doi.org/10.1021/jacs.1c11791
Written by He Jianchao
Rewritten by Wang Qinyuan
Edited by Shen Yutian and Hu Sijia