On March 6, 2019, Science Advances published online the latest research findings of Professor Du Haining’s research group on posttranslational modification and regulation of protein activity and function. This research reveals how the methylated modification of methyltransferase G9a exerts on Plk1, then regulates its kinase activity as well as the function that methylated modification exerts in repair of DNA damage.
The paper is entitled“A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair.” Doctoral candidates Li Weizhe and Wang Hongyan from the School of Life Sciences are first co-authors, as are Associate Professor Zhao Xiaolu, lecturer Tang Mingliang, and Professor Guo Lin, all from the School of Life Sciences. Other contributors include Professor Chen Qiang’s research group from the Medical Research Institute, Wuhan University, researcher Li Guohong’s team from the Institute of Biophysics, Chinese Academy of Sciences, Professor Liu Xiaoqi from Purdue University (now University of Kentucky). The research was conducted with support from the National Natural Science Foundation of China (NSFC) and National Protein Basic Research Program.
As a key cell cycle kinase, Plk1 modifies different protein substrates by phosphorylation to participate in regulating diverse biological function.
The protein level and kinase activity of Plk1 are strictly regulated, and the anomaly of protein level and kinase activity is strongly associated with the genesis of multifarious tumors. Previous research had found that the phosphorylation of the 210th threonine in protein Plk1 is the prerequisite to guarantee kinase activity to live up to its full potential during the mitotic phase, but the mechanism of how its kinase activity is kept under tight control during other cell cycles has yet to be confirmed.
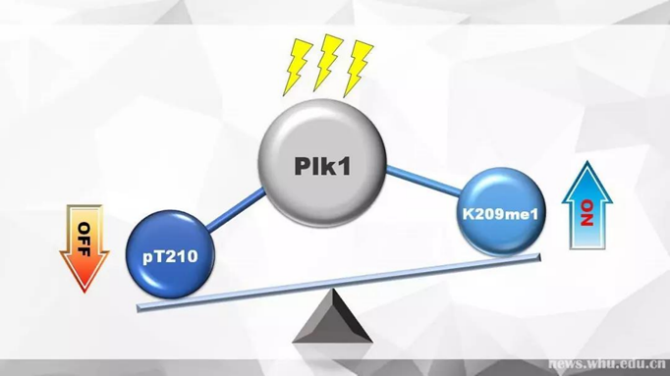
The translation of two levels of modification when DNA is damaged
(from official account of Graduate School of Wuhan University: Science Advances publishes research findings of Du Haining’s research group,3.13)
Utilizing the mass-spectrometric technique, Du Haining’s research group has authenticated that the 209th lysine near the 210th threonine can be methylated by G9a, and the two modifications dynamically controlled the kinase activity of Plk1 through mutual antagonism. Taking advantage of 209th lysine mutant cell line constructed by gene editing technology, the authors found that the continuous methylation on the 209th loci will lengthen the process of the cell’s mitotic phase remarkably. On the other hand, the ablation of the 209th locus’ methylated modification will induce cells to be more sensitive to DNA damage drugs, and the DNA damage cannot be repaired in time, indicating the critical role of the 209th locus’s methylated modification exerts in guiding the machinery of DNA damage repair.(from A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair, Science Advances 06 Mar 2019)This research not only reveals the elaborate regulatory mechanism of different posttranslational modification on protein Plk1, but also provides a fundamental basis for the clinical treatment of related tumors.
Bibliography:
A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair
Science Advances 06 Mar 2019
Vol. 5, no. 3, eaau7566
DOI: 10.1126/sciadv.aau7566
Rewritten by:Wu Buer,edited by Zhang Shiqi, Shen Yuxi, Shi Weiya and Hu Sijia