Professor Liang Kaiwei's team from the Tai Kang Medical School at Wuhan University (WHU) published a research paper titled Mechano-oncogenic Cytoskeletal Remodeling Drives Leukemic Transformation with Mitochondrial Vesicle-mediated STING Activation in the journal Cell Stem Cell.
The study reveals that the leukemia-associated transcription factor fusion protein CBFβ-SMMHC induces hematopoietic malignant transformation by remodeling the cytoskeletal network. This triggers intracellular mechanical force changes, mitochondrial constriction, and the generation of mitochondrial-derived vesicles (MDVs), which activate the STING-mediated inflammatory signaling pathway.
WHU's research result is published in Cell Stem Cell.
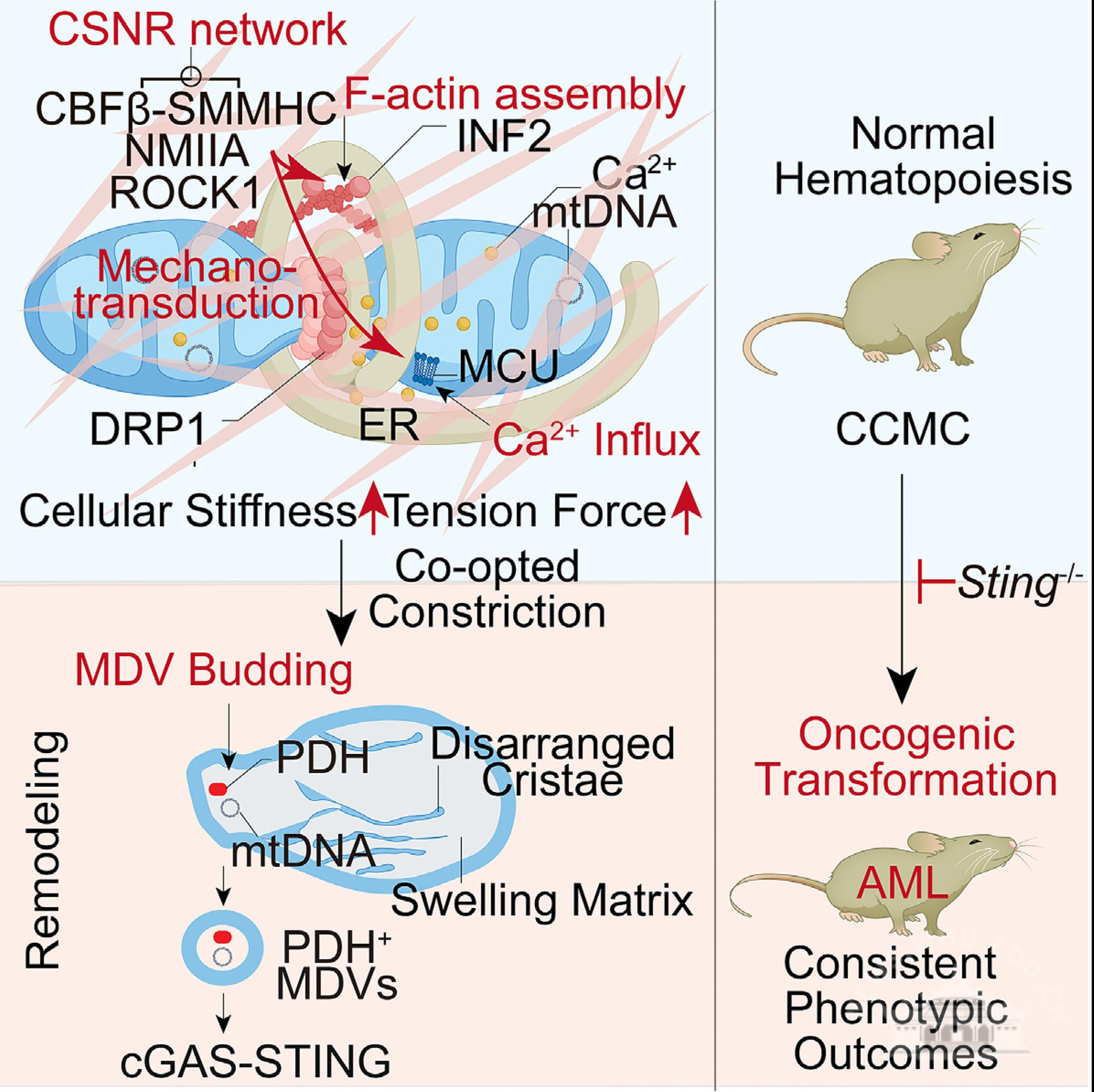
Mechanisms of leukemic transformation: cytoskeletal remodeling and mitochondrial vesicle-mediated sting activation.
Using techniques such as proteomics, the team discovered that the CBFβ-SMMHC fusion protein forms a stable complex with non-muscle myosin IIA and Rho kinase 1 (ROCK1), reconstructing a novel cytoskeletal network in the cytoplasm. This network collaborates with actin microfilaments to exert mechanical forces on mitochondria, inducing calcium ion influx and promoting mitochondrial inner membrane constriction, eventually leading to the formation of MDVs and activation of the STING pathway. This mechanism operates independently of the classical interaction between CBFβ-SMMHC and the RUNX1 transcription factor.
The study further validated this mechanism in a mouse leukemia model and found that similar cytoskeletal remodeling also induces transformation phenotypes in non-hematopoietic cells, suggesting the broader applicability of this mechanism across different tissues. The research team also developed a PROTAC degrader targeting the CBFβ-SMMHC fusion protein, combined with inhibitors targeting ROCK1 and STING, and found that this approach effectively delays leukemia progression, providing a new strategy for precision treatment of leukemia.
Link to paper: https://doi.org/10.1016/j.stem.2025.01.013