Professor Zhou Qianghui's research group at Wuhan University (WHU) recently published a paper titled Secondary Alkylation of Arenes via the Borono-Catellani Strategy in the Journal of the American Chemical Society. The paper presents an innovative method for synthesizing α-arylcoryl derivatives, marking an important advancement in the field of multisubstituted aromatic compound synthesis.

Zhou Qianghui's research team publishes a paper in the Journal of the American Chemical Society.
The research introduces a modular platform based on the Borono-Catellani aromatic secondary alkylation reaction. This reaction is characterized by mild conditions, broad substrate applicability, and excellent tolerance to various functional groups, with products exhibiting high diastereoselectivity (d.r. > 20:1). Additionally, the team achieved chiral secondary alkylation through dynamic kinetic asymmetric transformation (DyKAT), further expanding the diversity of the synthesis.
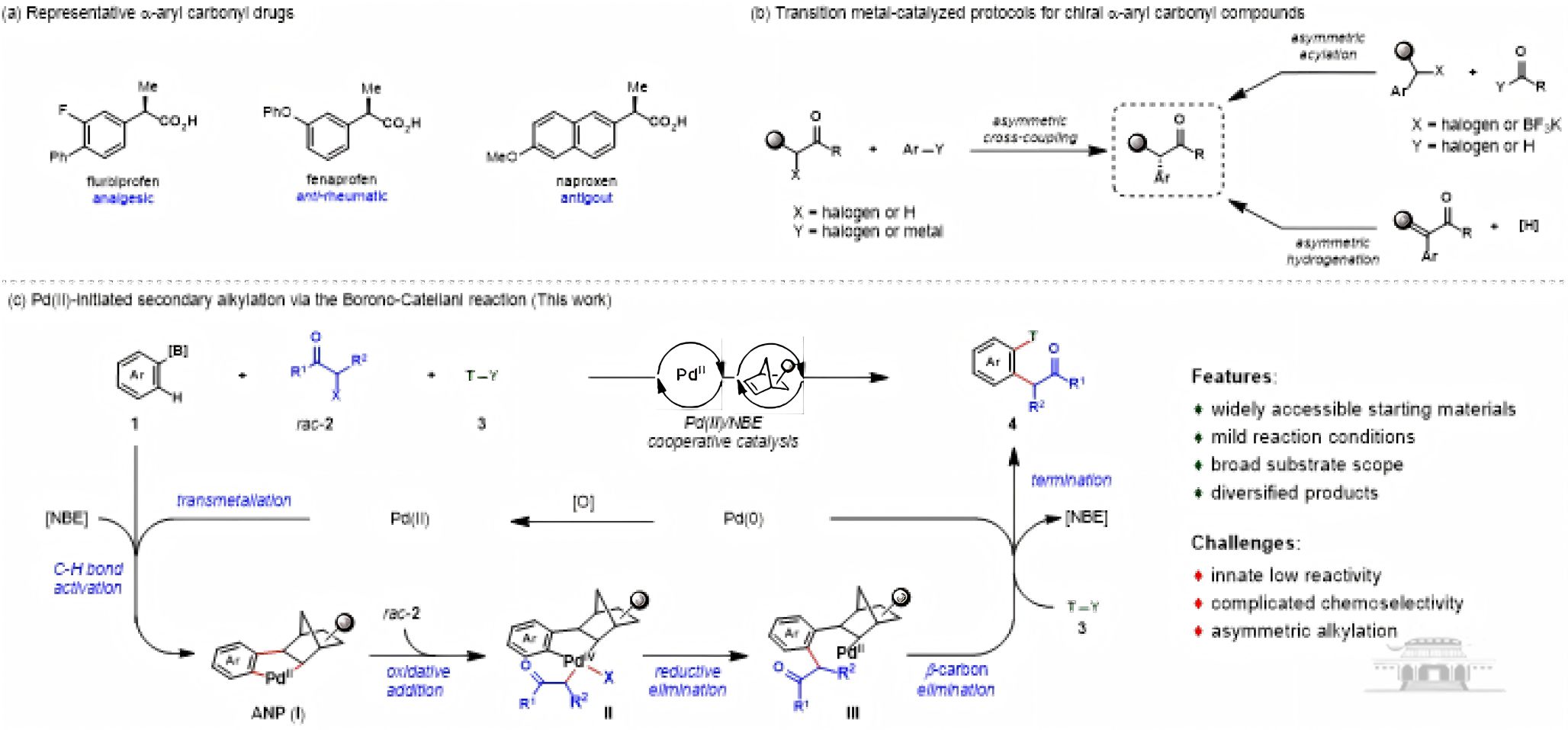
Palladium-catalyzed secondary alkylation of arenes via the borono-catellani strategy: A new approach to α-aryl carbonyl compounds.
The α-arylcoryl derivatives have wide applications in the pharmaceutical industry, with drugs such as flurbiprofen and fenoprofen playing key roles in pain relief and anti-rheumatism. Traditional synthetic methods often have limited product diversity and require harsh conditions. Therefore, this research provides a new pathway for the synthesis of novel pharmaceutical compounds.
Link to paper: https://pubs.acs.org/doi/10.1021/jacs.4c15956