A research team led by Professor Kong Wangqing at Wuhan University (WHU) has achieved a breakthrough in the stereoselective alkylation of C(sp³)-H bonds in saturated heterocycles. Their findings were recently published in Nature Chemistry.
Nickel-catalyzed enantioselective hydroalkylation for constructing vicinal c(sp³) centers.
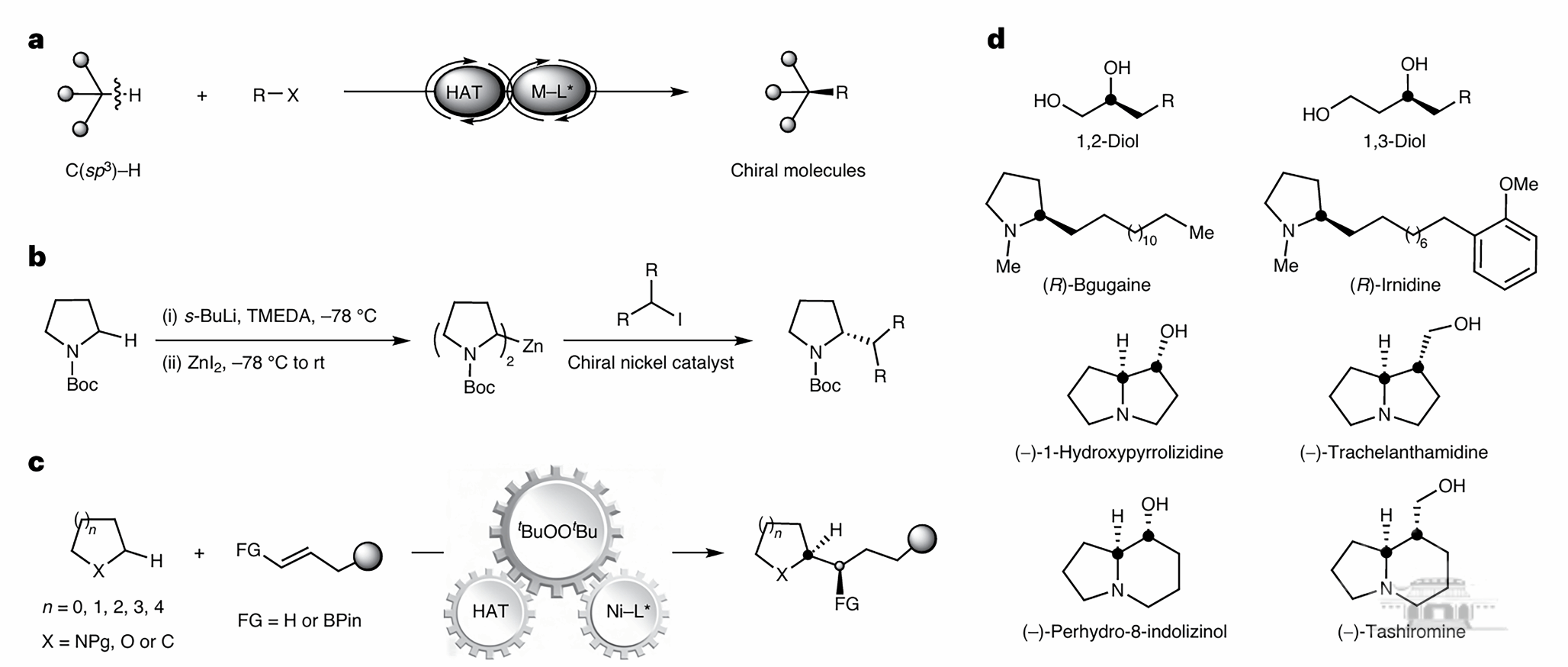
C(sp³)-H bond functionalization is a crucial approach for constructing chiral compounds widely used in pharmaceutical synthesis. However, existing methods often rely on expensive metal catalysts, high temperatures, or directing groups, posing challenges for broader application.
To overcome these limitations, the WHU team developed an innovative strategy that combines hydrogen atom transfer (HAT) with nickel catalysis, enabling enantioselective C(sp³)-H alkylation of saturated nitrogen- and oxygen-containing heterocycles.
The new method utilizes readily available alkenes as raw materials, operates under mild conditions, and exhibits a broad substrate scope. Additionally, it can be applied to the late-stage modification of natural products and drug molecules, opening new avenues for synthesizing chiral compounds.
Link to paper: https://www.nature.com/articles/s41557-025-01747-6