A groundbreaking study by Professor Liu Yibin and Professor Zhou Xiang from Wuhan University's College of Chemistry and Molecular Sciences, in collaboration with Professor Yu Zhixiang from Peking University's College of Chemistry and Molecular Engineering, has been published as an inside cover article in the prestigious Journal of the American Chemical Society (J. Am. Chem. Soc.).
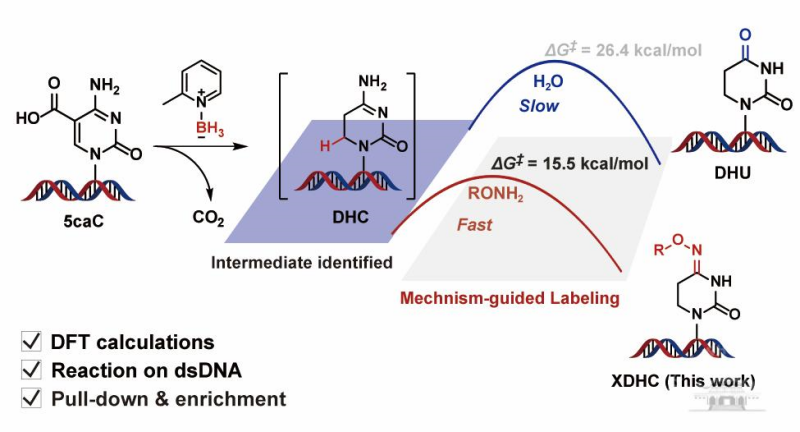
The paper, Mechanistic Insights into the Borane-Mediated Conversion of 5-Carboxylcytosine to Dihydrouracil in DNA Enable an Efficient Labeling Method Using Alkoxyamines, unveils a novel mechanism for converting 5-carboxylcytosine (5caC) to dihydrouracil (DHU) in DNA.
Using isotopic labeling, substrate derivatization, intermediate capture, and density functional theory (DFT) calculations, the research team elucidated that the conversion involves three key steps — reduction, decarboxylation, and hydrolysis — and identified, for the first time, a critical dihydropyrimidine (DHC) intermediate.
Both computational and experimental results demonstrated a "substrate catalysis" effect between adjacent 5caC bases on the DNA double helix, providing a robust theoretical foundation for efficient transformation on double-stranded DNA.
The researchers proposed an innovative "mechanism-guided labeling strategy" by introducing highly nucleophilic alkoxyamines to intercept the DHC intermediate during the rate-determining hydrolysis step to DHU.
This strategy significantly lowers the reaction energy barrier, shortens reaction time, and efficiently produces N4-substituted dihydropyrimidine (XDHC), which is read as T during sequencing.
The team also employed propargyl hydroxylamine via "Click" chemistry to achieve efficient enrichment and single-base-resolution detection of 5caC on double-stranded DNA. Using dot blot hybridization, they verified for the first time the presence of 5caC modifications in the DNA of human embryonic stem cells (hESCs).