The design approach for biomass electro-oxidation catalysts.
The prestigious journal Angewandte Chemie International Edition has published the latest research findings from Professor Deng Hongbing and Associate Researcher Li Wei's team at the School of Resource and Environmental Sciences, Wuhan University.
The paper, titled Activating Co2+/Co3+ Redox Couple Mediated-Electrooxidation of 5-Hydroxymethyl Furfuryl Under Low Potentials, explores the electrochemical oxidation of 5-hydroxymethylfurfural (eHMFOR).
This process enables the synthesis of 2,5-furandicarboxylic acid (FDCA), a high-value monomer for polyethylene furanoate (PEF) derived from biomass, under mild conditions. FDCA holds great potential in the polyester manufacturing industry.
The research enhances hydrogen production efficiency by providing a more energy-efficient pathway and advances the development of environmentally friendly materials, underscoring the critical role of eHMFOR in transitioning to sustainable resource-efficient economies.
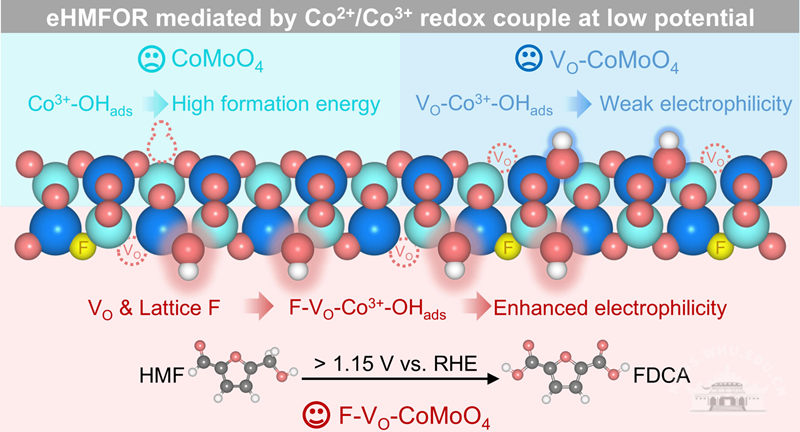
The research team designed an innovative F-VO-CoMoO4 catalyst, which features both oxygen vacancies (VO) and lattice fluorine (F) doping. The catalyst promotes the generation of *OH at low potentials through VO and enhances the electrophilicity of key intermediates with the strong electronegativity of F atoms, synergistically activating the Co2+/Co3+ redox couple for eHMFOR at low potentials.
The conversion of HMF to the high-value chemical FDCA is driven at a low potential of just 1.15 V, achieving a yield of 95.3 percent and a Faradaic efficiency of 96.1 percent. This breakthrough overcomes the performance limitations of traditional cobalt-based catalysts and provides a theoretical foundation for designing efficient biomass electro-oxidation catalysts.