A pioneering study by Professors Tian Tian and Zhou Xiang from Wuhan University's College of Chemistry and Molecular Sciences, in collaboration with Professor Liu Wenbo's team, has been published in Nature Communications.
The paper, titled A Catalyst-Free Bioorthogonal Reaction for Malononitrile Addition to Azodicarboxylates, introduces a novel bioorthogonal reaction system, MAAD (malononitrile addition to azodicarboxylates).
The MAAD reaction utilizes malononitrile and azodicarboxylates as its reactive pair. The reaction operates at room temperature under aqueous and physiological conditions without additional catalysts or additives.
It exhibits high selectivity and conversion rates across a broad pH spectrum and varying ionic strengths, even in complex environments containing glutathione and proteins. The ease of substrate synthesis and modification highlights its excellent biocompatibility and versatility.
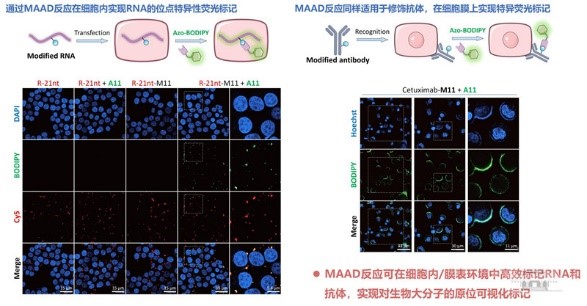
The research team explored diverse applications for the MAAD reaction. They employed 2'-OH modification reagents with malononitrile handles to achieve precise chemical labeling of RNA backbones.
The MAAD reaction also facilitates specific labeling and fluorescent tracing of proteins and cell membrane receptors, offering innovative strategies for antibody probes and membrane protein imaging.
One of the most innovative aspects of the research is the integration of the MAAD reaction into the CRISPR system. By modifying sgRNA/crRNA with malononitrile handles, the MAAD reaction enhances spatial effects at the chemical modification site, enabling precise regulation of Cas9 and Cas13a activity.
The MAAD reaction combines a novel reaction mode with high biocompatibility and excellent orthogonality, making it applicable in various scenarios such as nucleic acid and protein labeling and gene editing regulation.