Professors Wang Chunjiang and Dong Xiuqin from Wuhan University's College of Chemistry and Molecular Sciences have achieved a significant breakthrough in bimetallic sequential catalysis.
Their latest research, Stereodivergent Construction of Spiropyrrolidine-γ-Butyrolactones Enabled by Cu/Ru Sequential Catalysis and Stereoselective Reduction, has been published in the journal Angewandte Chemie International Edition.
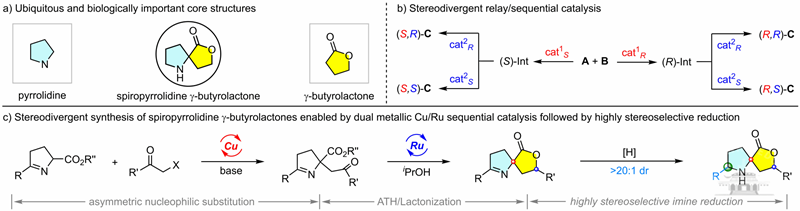
Sequential or relay catalysis offers distinct advantages by allowing independent stereochemical control over newly formed chiral centers across consecutive reaction steps. This method facilitates the stereodivergent synthesis of all possible stereoisomers from simple starting materials in a single-pot process, aligning with green chemistry principles.
Building on their previous successes in Cu/Ru relay catalysis using hydrogen-borrowing strategies, as documented in Angew. Chem. Int. Ed. 2022, e202206517 and Angew. Chem. Int. Ed. 2024, e202315325, Wang and Dong have now focused their efforts on synthesizing chiral spiropyrrolidine-γ-butyrolactone frameworks.
Utilizing chloroketone and cyclic imino esters as substrates, they constructed chiral spirodihydropyrrolidine-γ-butyrolactones containing two non-adjacent chiral centers. This was achieved through a cascade reaction involving asymmetric nucleophilic substitution, asymmetric transfer hydrogenation, and lactonization.
The process was further enhanced by a subsequent high-stereoselectivity imine reduction step, which introduced a third chiral center with excellent diastereoselectivity (>20:1 dr). This innovation resulted in a unique spirocyclic framework with three non-adjacent chiral centers, enabling the stereodivergent synthesis of all four stereoisomers.
These chiral spiropyrrolidine-γ-butyrolactones have demonstrated their potential as secondary amine catalysts. They have been applied in the asymmetric chlorination of aldehydes, Aldol condensation, and Michael addition reactions, demonstrating promising catalytic performance and broad potential applications.