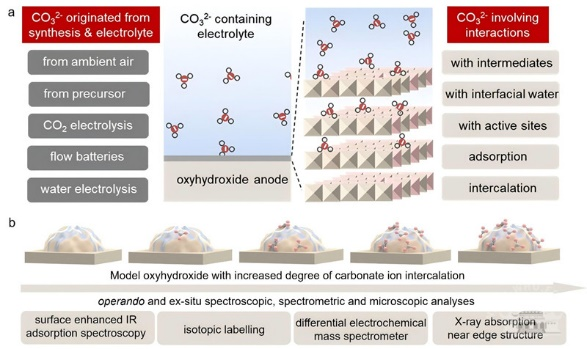
A diagram of the interaction between carbonate electrolytes and layered oxyhydroxides.
A research team led by Professor Yan Ning from the School of Physics and Technology at Wuhan University has achieved a significant breakthrough in water oxidation catalysis.
Their study, titled "Carbonate electrolytes manipulate lattice oxygen dynamics of oxyhydroxides toward efficient and durable water oxidation”, was recently published in the journal Nature Communications.
The research reveals how carbonate anions, a common component in electrolytes, can manipulate the reaction dynamics of lattice oxygen within catalysts. This manipulation enhances both the efficiency and stability of oxygen evolution reactions (OER).
The team explored the crucial role of carbonate ions in electrolytes and discovered that carbonates have a profound impact on the activity and redox stability of lattice oxygen.
The team uncovered how two distinct forms of carbonate influence the OER activity of oxyhydroxides through electrochemical cycling experiments, complemented by advanced isotope labeling and in-situ spectroscopic analysis.
Surface-adsorbed carbonates were found to enhance the oxygen evolution activity of NiOOH, while intercalated carbonates reduced the activity of CoOOH and NiCoOOH. The finding enabled the researchers to balance the "release" and "refill" of lattice oxygen during reactions by controlling carbonate ion intercalation.
The discovery paves the way for designing more efficient and durable catalytic systems, accelerating the adoption of renewable energy solutions.