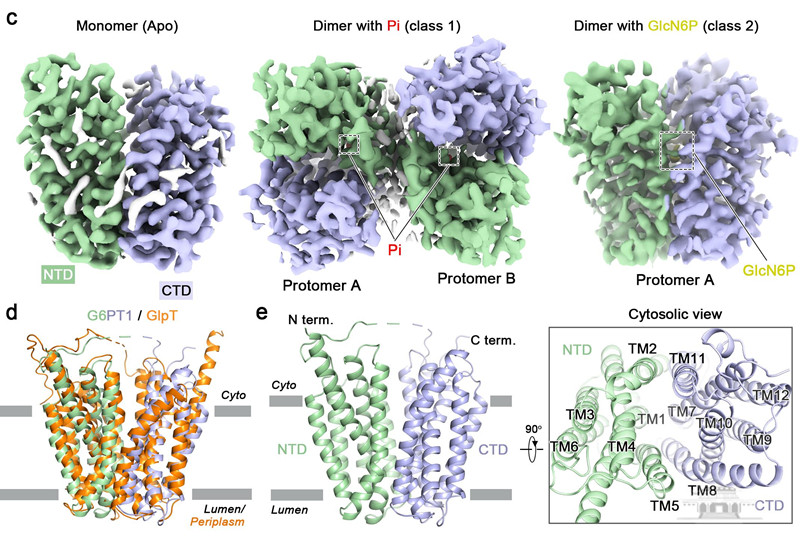
Cryo-EM structure determination of human G6PT1.
A groundbreaking study has been published in Nature Communications by a collaborative team from Wuhan University's School of Pharmaceutical Sciences, led by Gao Shuai and Yao Xia, alongside Liu Lei's team from Tsinghua University.
The research, Structures of human glucose-6-phosphate transporter reveal reciprocal antiport mechanism driving glucose-6-phosphate and inorganic phosphate exchange, provides new insights into the transport mechanism of the human glucose-6-phosphate transporter (G6PT1).
The team captured high-resolution cryo-EM structures of human G6PT1 (~46 kDa) in its Apo, Pi-bound, and GlcN6P-bound states (a G6P analog) using advanced techniques, including single-particle cryo-electron microscopy and biochemical methods.
The study also clarified the molecular basis for the recognition of Pi and G6P within G6PT1 through molecular docking and functional assays. Comparative analysis revealed that Pi binding induces the formation of a cross-domain salt bridge, thickening the lumenal gate and compacting the central cavity to accommodate G6P binding.
The study also resolved the high-resolution structure of the G6PT1 dimer. Mutations at key residues within the dimer interface significantly impaired transport activity, suggesting that the protein's oligomerization state is crucial for its functional regulation.
This research provides a structural framework crucial for understanding the operational mechanism of G6PT1 and its involvement in the pathological dysregulation seen in glycogen storage disease type Ib (GSD1b).