The catalytic mechanism and role of strongly electron-accepting lattice oxygen in CeO₂.
A team led by Professor Ding Mingyue of the School of Power and Mechanical Engineering at Wuhan University has published a groundbreaking study in Angewandte Chemie International Edition.
The paper, Strongly Electron‐Accepting Lattice Oxygen of CeO₂ for Highly Efficient Dimethyl Carbonate Synthesis from CO₂ and Methanol, reports significant advances in the synthesis of dimethyl carbonate (DMC) from CO₂ and methanol.
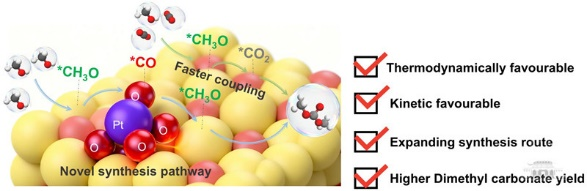
Transforming CO₂ into high-value chemicals is essential for advancing green chemistry and achieving dual carbon goals. The direct synthesis of DMC from CO₂ and methanol has its advantages, but is hindered by thermodynamic non-spontaneity and high kinetic barriers, resulting in DMC yields of less than 1 percent.
The team developed a Pt single-atom-embedded CeO₂ catalyst (Pt1/CeO₂–SO) using a hydroxylation-induced Pt spontaneous redispersion strategy. This approach reconstructs the lattice oxygen coordination structure to Pt-*O-Ce, significantly enhancing its electron-accepting capability.
This facilitates the formation of highly active *CO intermediates, triggering a thermodynamically favorable *CH3O-*CO coupling pathway for DMC synthesis.
The electron modification of lattice oxygen also optimizes the catalyst's charge distribution, regulating the adsorption and activation of CO₂ and methanol.
The approach reduces the *CH3O-*CO₂ coupling barrier, altering the rate-determining step of DMC synthesis and accelerating the reaction rate. After optimizing reaction conditions, the Pt1/CeO₂-SO catalyst achieved a DMC yield of 62.1 mmol/g, a sixfold increase over traditional catalysts.
The study underscores the pivotal role of lattice oxygen modification in revolutionizing DMC synthesis pathways and optimizing reactant adsorption and activation, providing theoretical guidance and new insights for the efficient conversion of methanol and CO₂ and paving the way for future advancements in sustainable chemistry.