Professor Gao Lei from Wuhan University, in collaboration with Professor Lei Xiaoguang's team from the College of Chemistry and Molecular Engineering at Peking University, has published the latest advancements in enzymatic amide synthesis in Science.
The paper, Engineered aldehyde dehydrogenases for amide bond formation, presents a novel approach to constructing amide bonds, which are crucial structural units in both macromolecular proteins and small-molecule drugs.
Amide compounds have been synthesized from carboxylic acids, a process that often involves the consumption of stoichiometric condensing agents and the production of significant by-products. This method suffers from substrate limitations and poor atom economy.
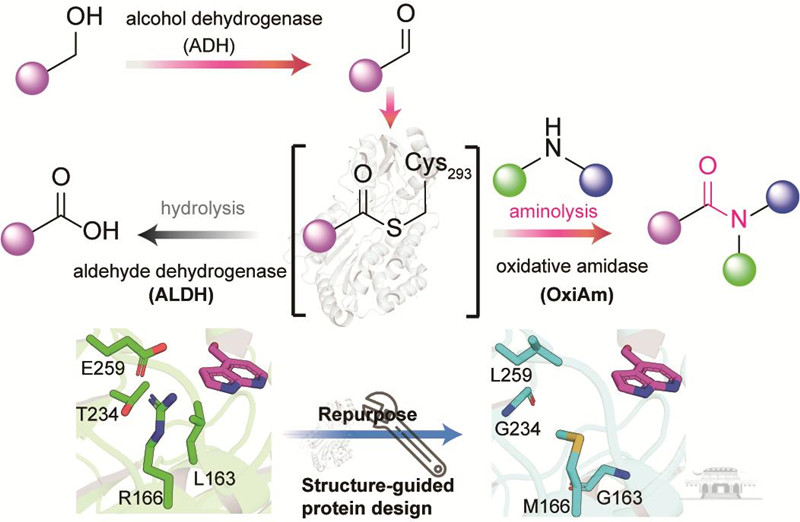
Aldehyde dehydrogenases (ALDHs), which are widespread in nature and use NAD(P)+ as a cofactor, play a vital role in converting structurally diverse aldehydes into carboxylic acids — crucial for energy and material metabolism in living organisms.
The research team, drawing on an in-depth understanding of enzymatic mechanisms, ingeniously modified para-hydroxybenzaldehyde dehydrogenase (PHBDD) from Pseudomonas putida.
This modification prevents hydrolysis of the thioester intermediate and instead promotes a specific aminolysis reaction with amines to form amides. Consequently, PHBDD was transformed into an oxidative amidation enzyme (OxiAm) capable of directly catalyzing the formation of amides from aldehydes and amines.
This engineered oxidative amidation enzyme exhibits remarkable substrate versatility and catalytic efficiency. It breaks the traditional paradigm of amide compound synthesis and significantly enhances the efficiency of amide drug synthesis, offering substantial industrial application potential.
This novel enzymatic tool can be coupled with alcohol dehydrogenase and NADPH oxidase, enabling a cascade reaction system that synthesizes amide compounds from lower-oxidation-state alcohols using inexpensive, readily available oxygen as an oxidant.
The team discovered that this protein engineering approach is broadly applicable; other ALDHs with the same mutations can also catalyze the reaction between aldehydes and amines to produce amides, opening a new direction for enzymatic amide bond formation.
The team has developed a transformative method for forming amide bonds from low-oxidation-state precursors, providing new pathways and tools for the green biomanufacturing of amide-based drugs.