On February 10th, Cancer Cell published an article entitled Direct Phosphorylation and Stabilization of MYC by Aurora B Kinase Promote T-cell Leukemogenesis. This research is co-completed by Prof. Liu Hudan and her team from Medical Research Institute, Wuhan University. In the article, they analyzed the molecular mechanism of how the new phosphorylation of oncogenic MYC promotes T-cell acute lymphoblastic leukemia (T-ALL) and proposed the new potential therapy for T-ALL via MYC intervention.
T-ALL is a highly malignant blood tumor and its current clinic treatment mainly depends on high-dose chemotherapy. In spite of a high recovery rate among children, they may endure the toxic and side effects caused by intense chemotherapy for a lifetime, accompanied with probable recurrence. What’s more, the poor prognosis among adult patients is common due to the intolerability of high-intensity chemotherapy. Therefore, it is in urgent need to develop a targeted therapy with high-efficiency and low toxicity. The new potential cure for T-ALL may target oncoprotein MYC, which is a critical driving force in the development of T-ALL. However, loose protein structure of MYC leads to a lack of functional domain suitable for micro-molecule binding, making it difficult to make a direct target therapy. Therefore, indirect target is the only method to resort to.
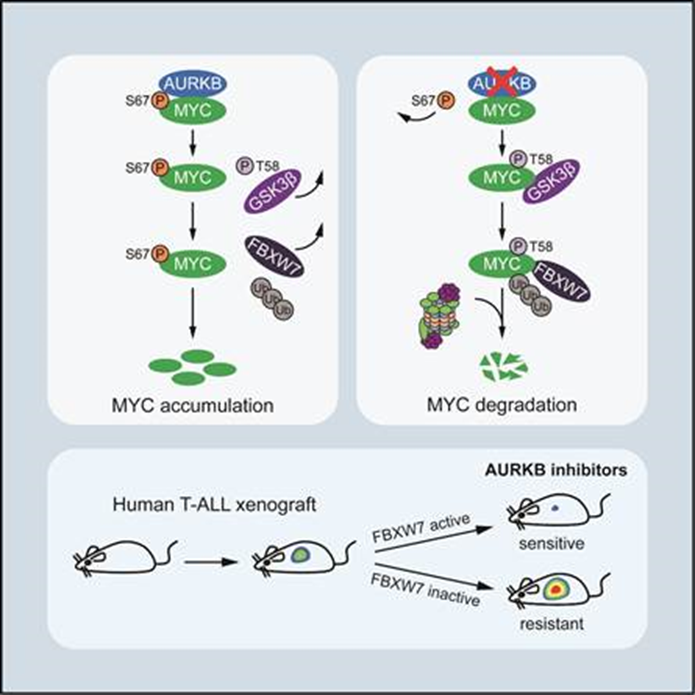
Liu Hudan’s group previously conducted proteomics screening and found that Aurora B kinase (AURKB) could directly bind to MYC and phosphorylate MYC-67 serine residues (S67). Their further study indicated that the phosphorylation of S67 breaks the interaction between MYC and GSK3β, thus inhibiting the phosphorylation of MYC-58 threonine (T58) and the recognition of an E3 ubiquitin ligase named FBXW7. As a result, the stability of MYC protein is significantly enhanced and its carcinogenic effect can be enlarged when MYC directly activated the gene transcription of AURKB to form a positive feedback loop.
Further, the research team made the transgenic zebrafish model of T-ALL to confirm that the positive feedback control of AURKB-MYC essentially influences the development of T-ALL. Then, after referring to the FDA-authorized library of micro-molecule compounds, the researchers found that Vincristine, the prior clinical drug of T-ALL, in cooperation with AZD1152, an AURKB depressor, can play a significant role against leukemia. In particular, this drug combination only selectively kills T-ALL of wild FBXW7, but is hardly useful for T-ALL with inactive and mutational FBXW7, which suggests that the gene mutation of FBXW7 can be used as a potential biomarker of the drug combination to treat T-ALL.
It is worth mentioning that the traditional theory holds that MYC-62 serine (S62) phosphorylation increases in proportion to the enhancing stability of MYC protein, while it also assists MYC to join with GSK3β, mediate T58 phosphorylation as well as MYC ubiquitin degradation. In this study, Prof. Liu Hudan and her team proposed a new mechanism that phosphorylated MYC S67 antagonizes GSK3βmerging into MYC so as to enhance the stability of MYC protein. They also proved the important function of phosphorylated S67 that showed in promoting the development of T-ALL.
Prof. Liu Hudan is the corresponding author of the article, and three co-first authors are PhD. Candidate Jiang Jue, PhD. Candidate Wang Jingchao from Medical Research Institute, Wuhan University, and Dr. Yue Ming from the Central Hospital of Wuhan affiliated to Huazhong University of Science and Technology. The research is supported by the National Key Research and Develop Program and the National Natural Science Foundation of China (NSFC).
Rewritten by: Zhu Tong