Recently, the prestigious international oncology journal Molecular Cancer (IF=15.302) published a review paper on extracellular vesicles and tumor immunity authored by the research team led by Prof. Li Dejia and Associate Prof. Sun Chengcao (School of Health Sciences, WHU) and Associate Prof. Tan Gongjun (Jinan University).
The paper is entitled "Comprehensive Landscape of Extracellular Vesicle-Derived RNAs in Cancer Initiation, Progression, Metastasis and Cancer Immunology". Hu Wei, a 2017 graduate student, Liu Cong, a 2016 doctoral student (School of Health Sciences, WHU), and Dr. Bi Zhuoyue (Hubei Provincial Academy for Preventive Medicine) are joint first authors. Sun Chengcao, Li Dejia, and Tan Gongjun are co-corresponding authors.
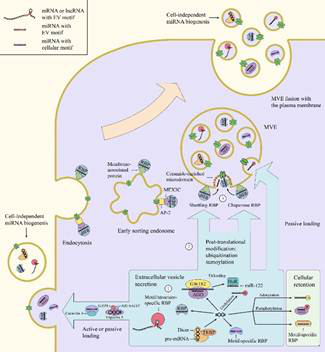
The process and regulatory mechanism of intracellular RNA entering EVs
Extracellular vesicles (EVs), a class of heterogeneous membrane vesicles, are generally divided into exosomes and microvesicles on the basis of their origination from the endosomal membrane or the plasma membrane, respectively. EVs can pass through the extracellular space and reach the recipient cell, activating the receptor on the surface of the cell or releasing the molecule it carries through internalizing or fusing with the cell, thereby transmitting its content or signal. Various types of RNA have been found in EVs, among which non-coding RNA, especially tiny non-coding RNA, accounts for the vast majority of EVs RNA. Intracellular RNAs are kept in close proximity to the site of EV biogenesis and then incorporated into the EV lumen, which is affected by the affinity of RNAs and their carriers to membrane lipids and proteins at the budding microdomain.
The abnormal regulation of EVs RNA in different cancers and its cell-specific functions have recently been revealed. EVs RNA transmit the information of donor cells to stromal cells or tumor cells in the vicinity or far away, promoting the crosstalks of cells in the tumor microenvironment. Cancer-derived EVs RNA-mediated angiogenesis, lymphangiogenesis, and increased vascular permeability can promote the generation of microenvironments before cancer cell proliferation and metastasis.
-Hypoxia is considered as a major driving force for shaping the tumor microenvironment (TME) and induces most, if not all, of cancer-malignant phenotypes by increasing tumor-promoting EV-RNA release. Hypoxia-induced cancer progression mediated by EV-RNAs is attributed to protumoral niches fostered by normal cells, endothelial permeability, angiogenesis, malignant evolution within tumors and immunosuppressive microenvironment. EVs RNA derived from cancer cells is involved in regulating the function of immune cells and secretion of cytokines, mediating immune reprogramming, thereby regulating anti-tumor immune responses and immune evasion. Tumor-promoting EV-RNAs are involved in the cancer cell-stromal cell, immune cell-immune cell and cancer cell-cancer cell crosstalks, thereby promoting the initiation, growth, angiogenesis and survival of primary tumor, as well as multiple steps of the metastatic process, including local invasion, intravasation, extravasation and outgrowth of cancer cells at metastatic sites.
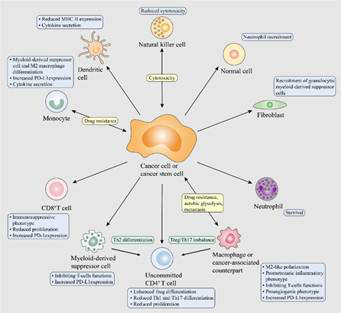
EV-RNA mediated crosstalk within immune cells and between immune cells and cancer cells modulating malignant behaviors of cancer cells
The review paper systematically summarizes the current findings regarding EV biogenesis, release and interaction with target cells as well as EV-RNA sorting, and highlight biological roles and molecular mechanisms of EV-ncRNAs in cancer biology. It pointed out that EV-RNAs regulate both innate and adaptive immune systems of the host by participating in cancer cell-immune cell and immune cell-immune cell crosstalks. Also, it depicts the direct relationship among EVs RNA, tumorigenesis and tumor immunity, and potential therapeutic target.
DOI: https://doi.org/10.1186/s12943-020-01199-1
Written by: Sun Chengcao
Rewritten by: Dong Xiaoqian
Edited by: Wu Buer, Sylvia and Hu Sijia