On August 10, the top international journal Nature Catalysis (IF: 30.47) published online the latest findings on the efficient construction of biaryl compounds with axial chirality via catalytic asymmetric Catellani reaction by Prof. Zhou Qianghui’s research group. Prof. Zhou, from College of Chemistry and Molecular Science, is also a researcher at the Institute for Advanced Studies at Wuhan University (WHU).
The paper is entitled Construction of axial Chirality via Palladium /chiral Norbornene Cooperative Catalysis. Postdoctoral fellow Liu Zeshui is the first author, and Zhou Qianghui is the corresponding author. The research was supported by the National Natural Science Foundation of China and received start-up funding from WHU.
Axial-chiral biaryls are common and important structural units in bioactive natural products, pharmaceutical molecules, materials and chiral catalysts/ligands. Therefore, the development of a concise and general method for the synthesis of axially chiral biaryl compounds is of great importance.
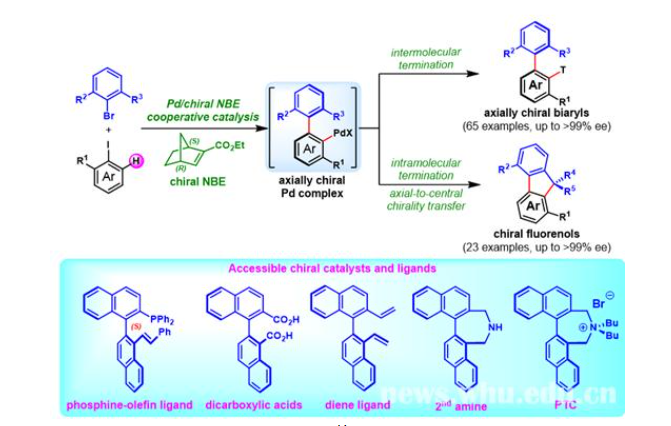
Figure 1: Construction of axially chiral biaryls and chiral fluorenols via Pd/chiral norbornene
cooperative catalysis
Prof. Zhou’s team has been working on efficient organic synthesis. Based on previous research, they developed a novel modular platform technology for the construction of axial chirality via catalytic asymmetric Catellani reaction. Reactants include readily available aryl iodides, aryl bromides and olefin (or alkynes, boronic acid, etc.). Through palladium/chiral norbornene cooperative catalysis, an assortment of axially chiral biaryls can be obtained in maximum yields of 96% and enatiomeric excesses up to 99% (88 examples).
This catalytic method involves low-cost and easily available reactants, widely applicable substrates, and boasts high chemoselectivity, excellent enantioselectivities and scalability. More importantly, the axial-chiral biaryl products can be readily converted into useful chiral ligands and catalysts. For example, chiral phosphine-olefin ligand prepared after a one-pot conversion exhibits high reactivity and good enantioselectivity for palladium-catalysed asymmetric allylations.
In addition, the intermolecular termination of axial-chiral palladium intermediate obtained can contribute to axial-to-central chirality transfer and thus to the efficient construction of chiral fluorenols. It is a new synthetic method (figure 1) and enantioselectivity is up to 99%.
It is worth mentioning that the brand-new research field of catalytic asymmetric Catellani reaction entails many challenges, with only a few studies reported so far. Palladium/chiral norbornene is chosen as a co-catalyst to construct the axial chirality. The use of simple chiral norbornene as the sole chiral source achieves the de novo construction of the axial chirality, which effectively makes up for the deficiencies of existing strategies and boosts the development of catalytic asymmetric Catellani reaction.

Figure 2:
A group photo of some members of this project, including Zhou Qianghui (first from the left in the back row) and Liu Zeshui (first from the right in the back row)
Article Link: https://www.nature.com/articles/s41929-020-0494-1
Home page of Zhou Qianghui’s research team: http://qhzhou.whu.edu.cn
Written by: Hua Yuan
Rewritten by: Shi Shang
Edited by: Dong Xiaoqian, Sylvia and Hu Sijia